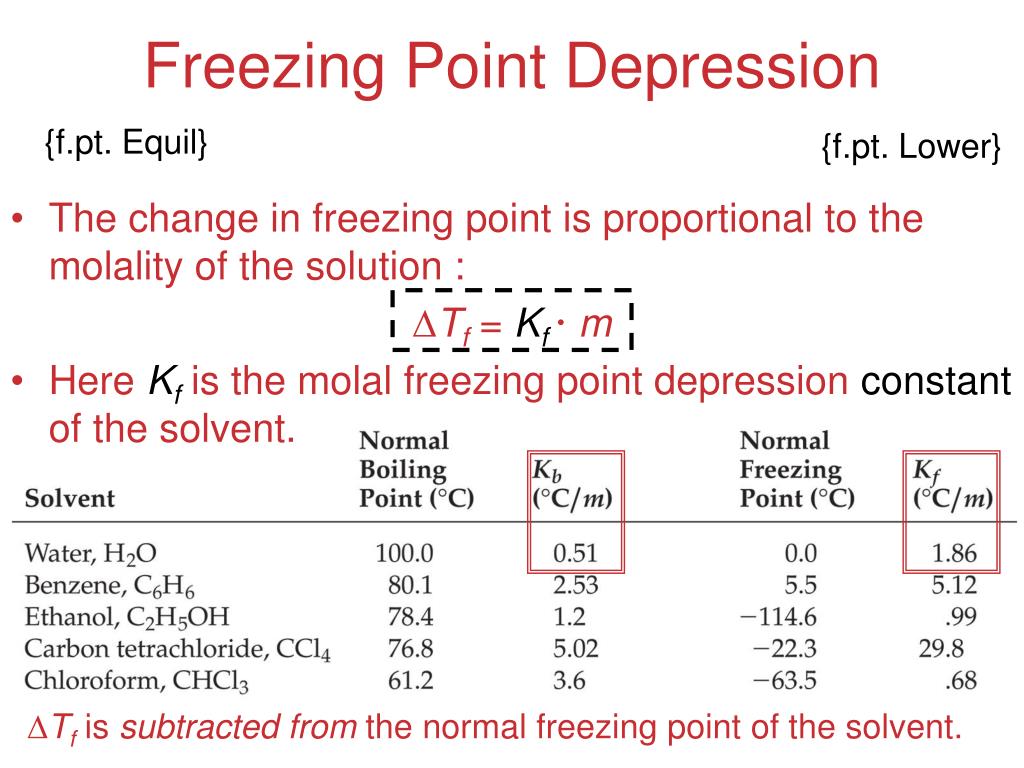
Freezing Point Depression
Get Freezing point depression For Android - Mobile Wallpapers — Candidly Keri. Candidly Keri is a place for reviews, makeup, clothes, advice and designs of wallpapers and. Today i will share Freezing point depression wallpaper. From removable abstract wall murals to unique wallpaper with designs you have never seen before, these are the best places to buy trendy home.
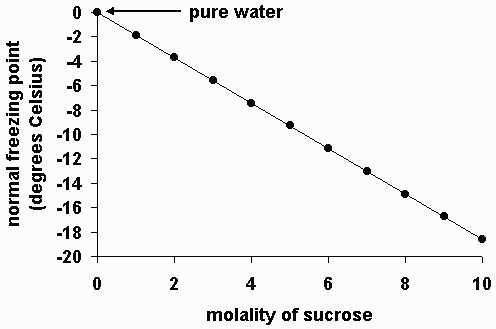
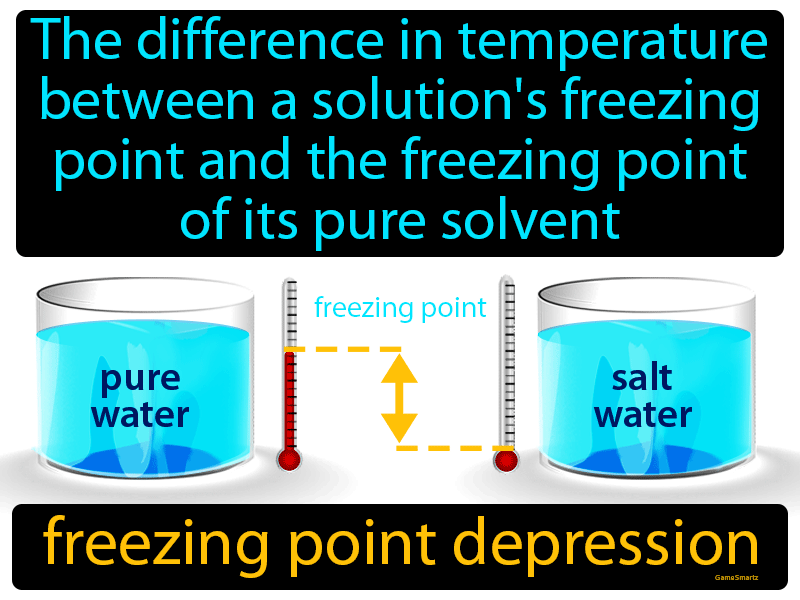
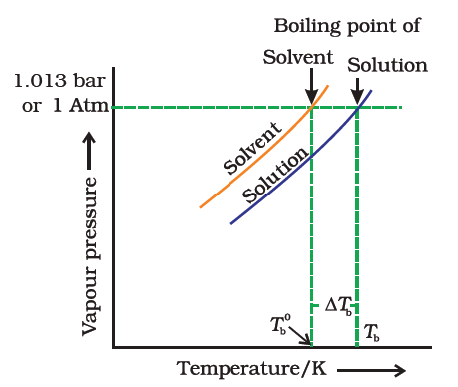
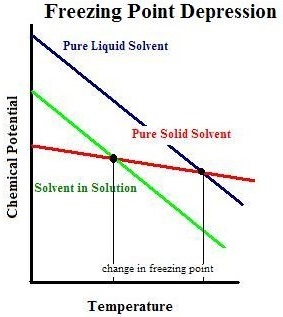
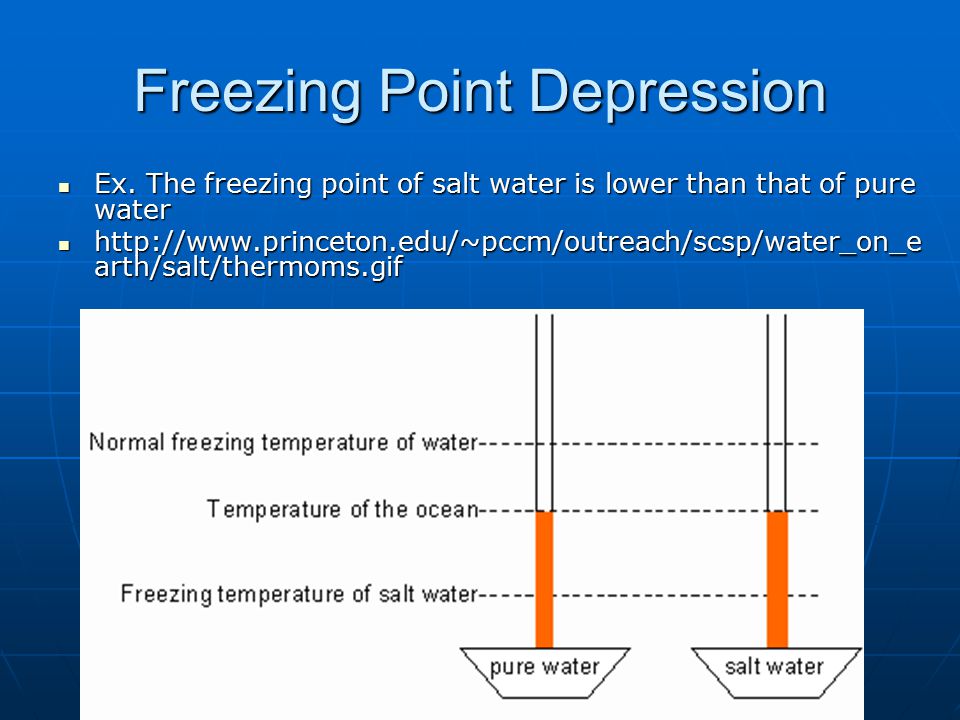
The freezing point of a solution is less than the freezing point of the pure solvent.
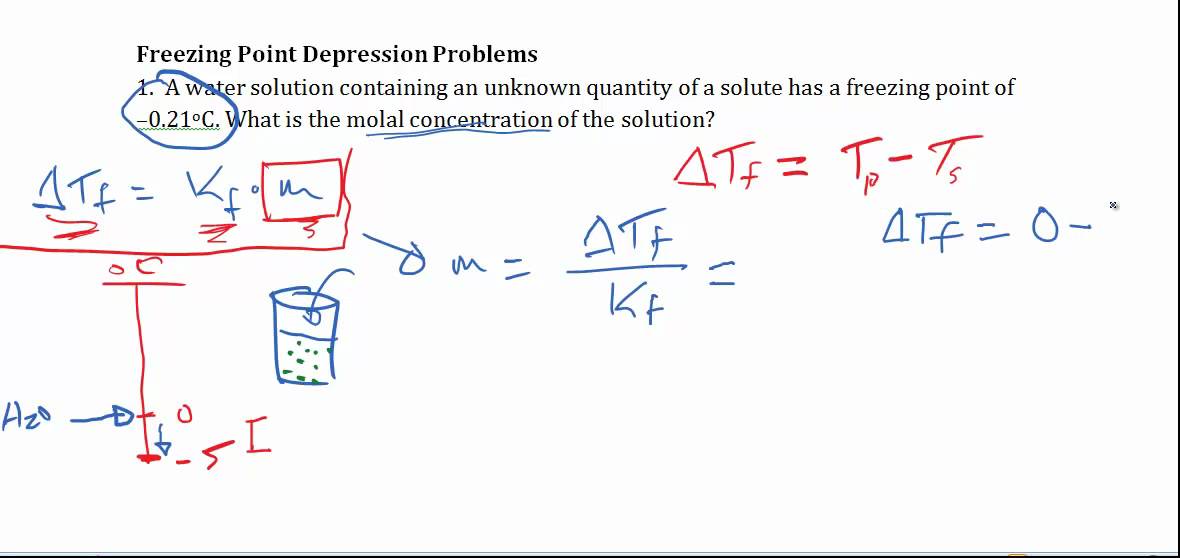
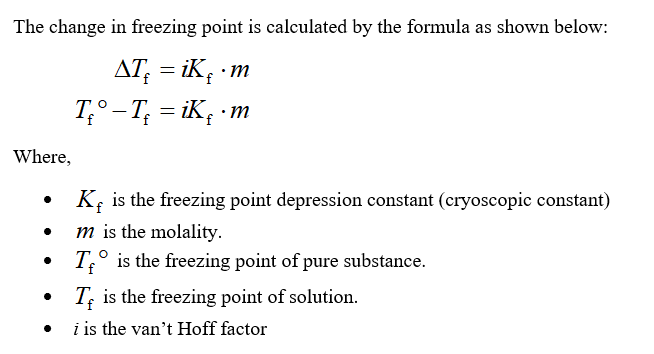
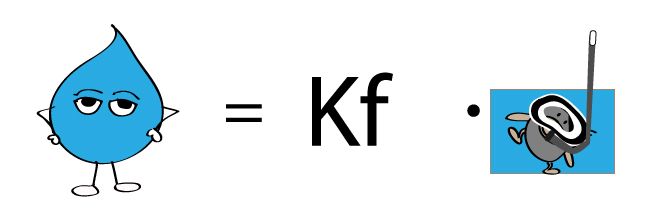
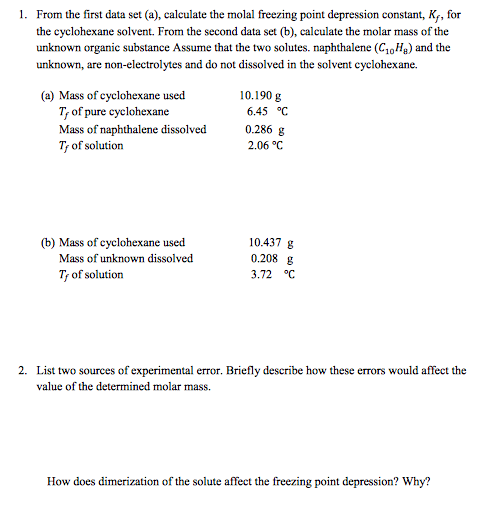
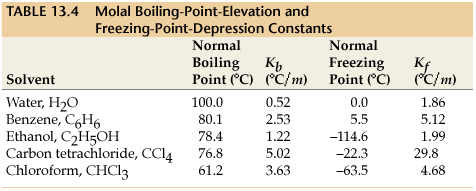
Deltatf tfsolvent tf solution kf times m.

Download Freezing point depression Free HD
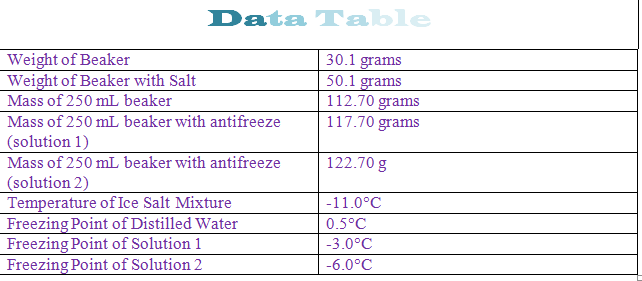
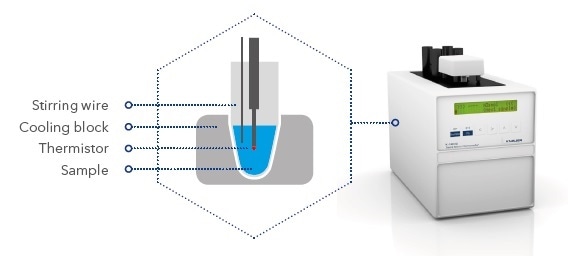
Freezing point depression. The osmometry is the most preferred in among other areas pharmaceutical quality control laboratories and in clinical chemistry. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. Uses of freezing point depression.
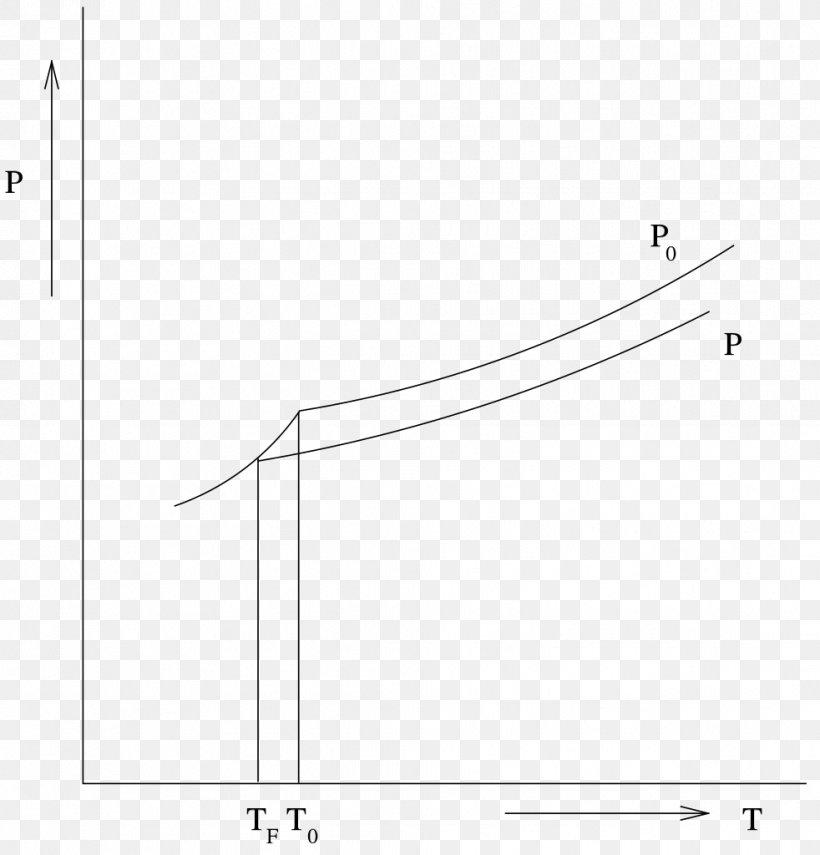
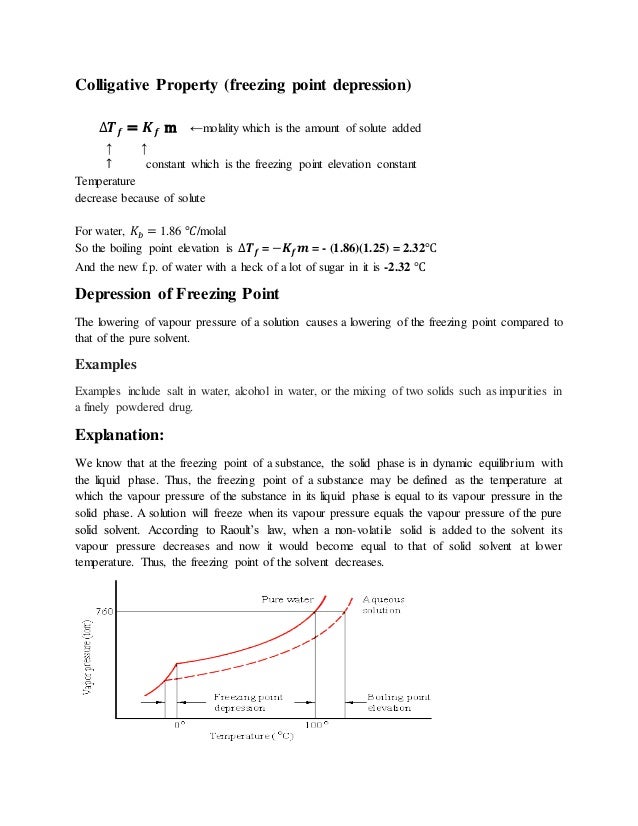
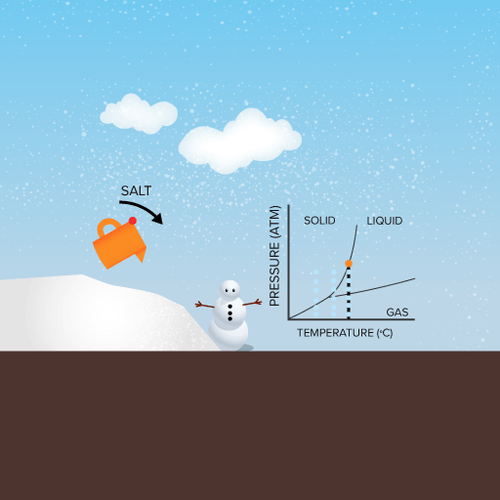
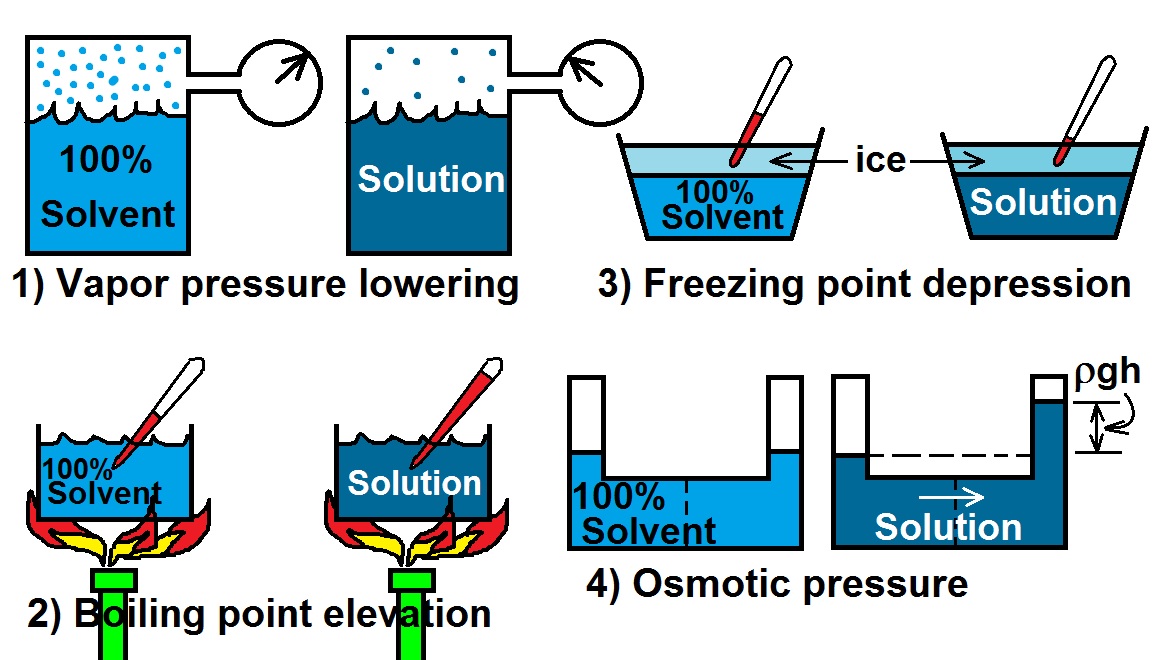
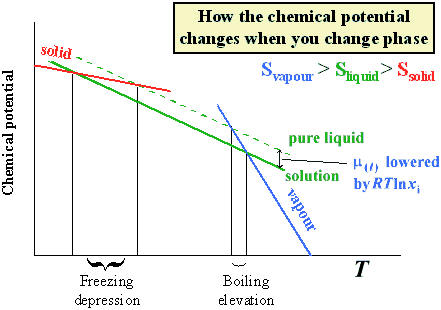
At the freezing point the vapor pressure of both the solid and liquid form of a compound must be equal. Instead it only depends on the number of particles added as well as the original properties of the solvent to which they are added. Freezing point depression is the decrease of the freezing point of a solvent on the addition of a non volatile soluteexamples include salt in water alcohol in water or the mixing of two solids such as impurities into a finely powdered drug. The freezing point depression can be calculated using the pure solvent freezing point and the molality of the solution.
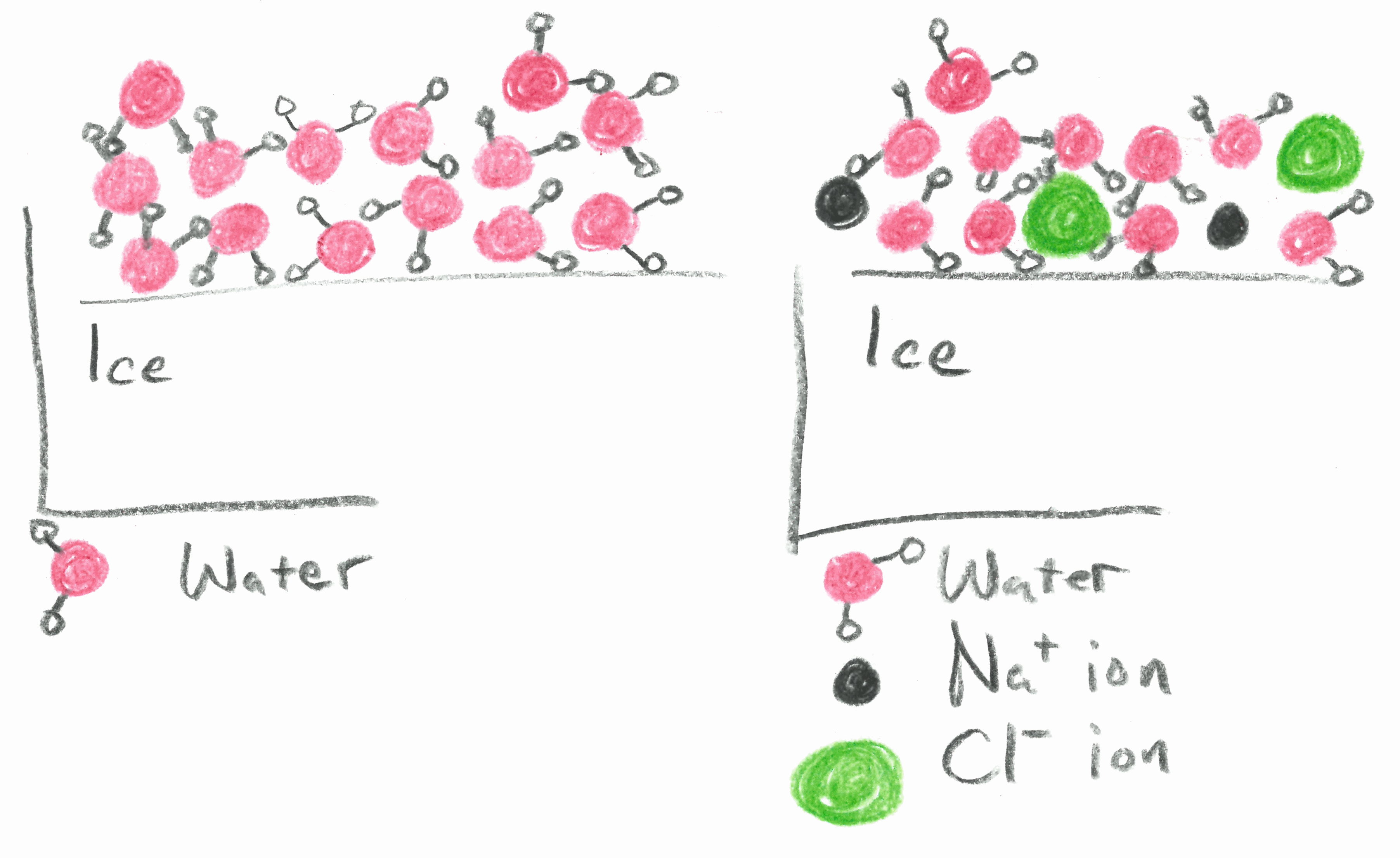
Freezing point depression is one of the colligative properties of matter which means it is affected by the number of particles not the chemical identity of the particles or their mass. It doesnt matter whether the solute is a. If the temperatures are below 18 o c calcium chloride is used instead of nacl to melt the ice on the roads. The freezing point depression is a so called colligative property.
Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. Freezing point depression osmometry is however the most preferred in distinct contexts. The vapor pressure of a solution blue is lower than the vapor pressure of a pure solvent pink. Some important uses of freezing point depression are listed below.
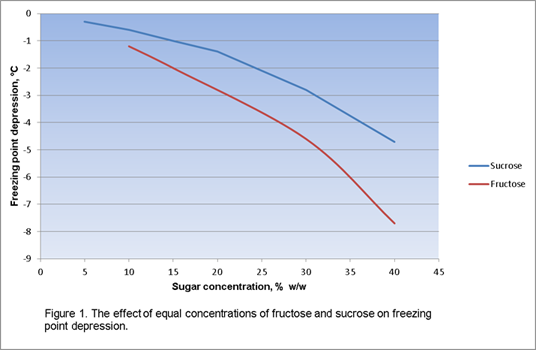
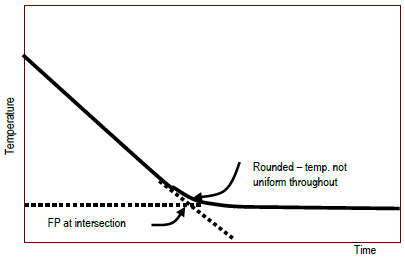
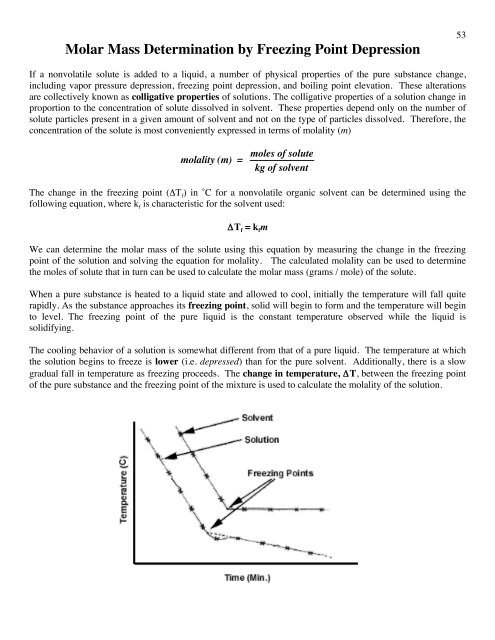
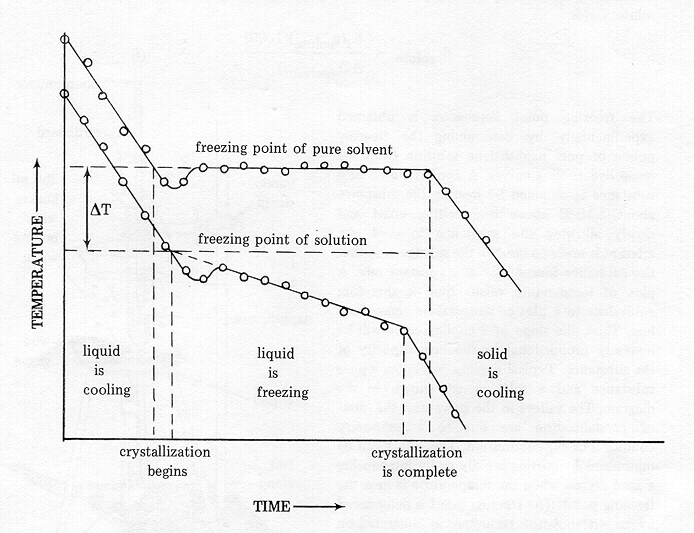
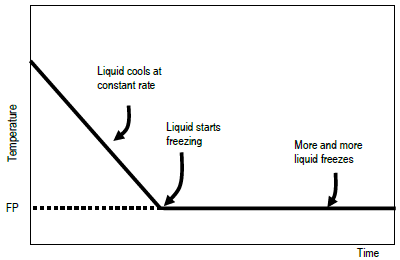
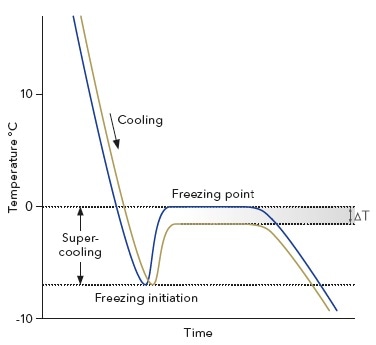
Colligative properties depend on the number of particles present not on the type of particles or their mass. On the graph the freezing point depression is represented by. The freezing point of a substance is the temperature at which the solid and liquid forms are in equilibrium. In the past it has been used to assess the osmotic strength of a colloid and solutions.
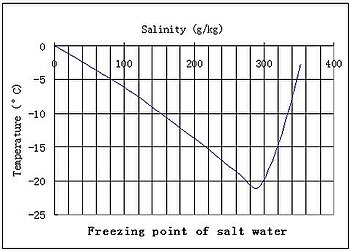
As a result the freezing point of a. So for example if both calcium chloride cacl 2 and sodium chloride nacl completely dissolve in water the calcium chloride would lower the freezing point more than the sodium chloride because it would produce three. When a solute is added to a solvent its freezing point is lowered from the original value of the pure solvent. Freezing point depression is a colligative property of matter.
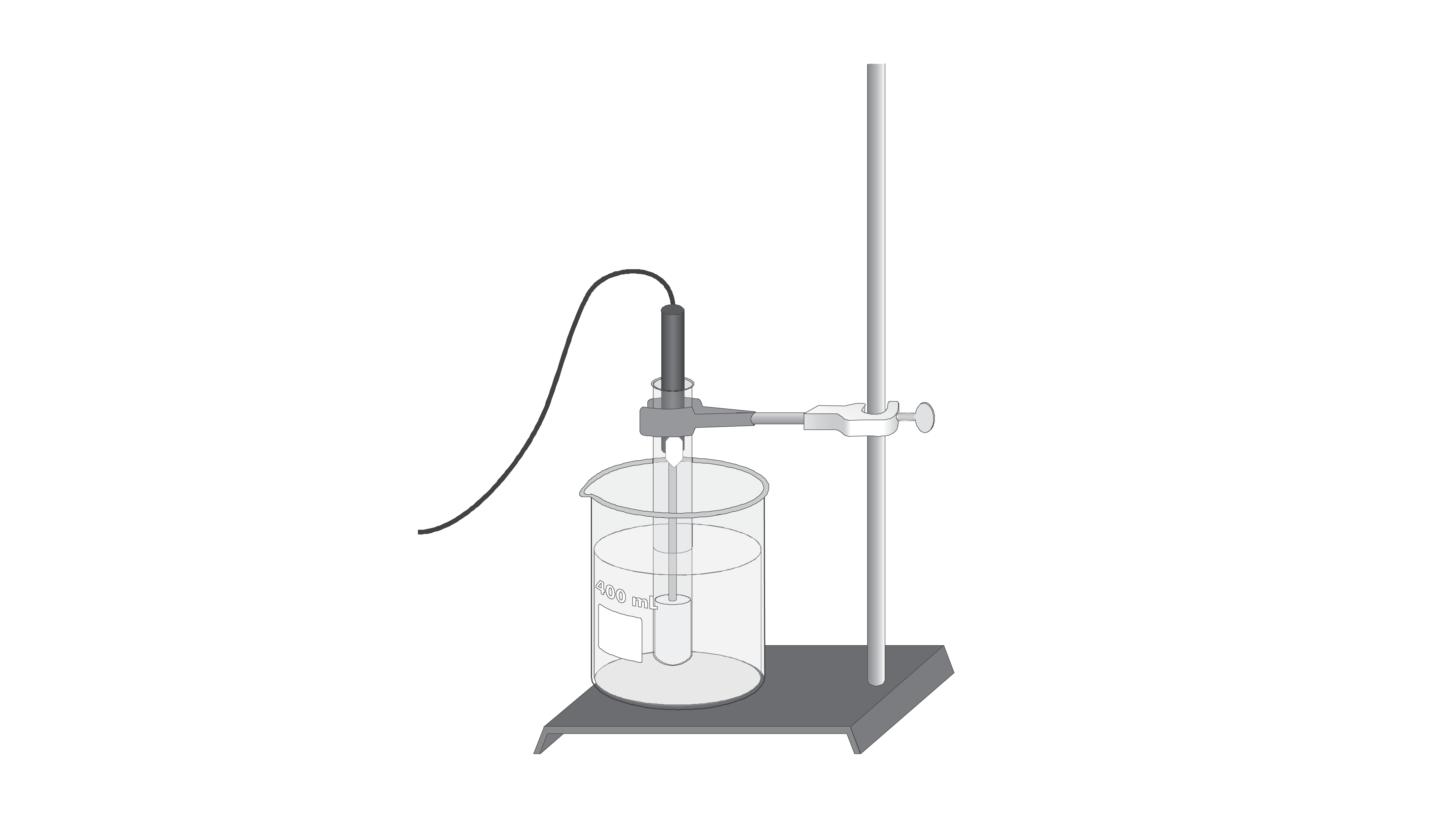
This means that the temperature drop so how much the freezing point lowers does not depend on the type of component added the solute. The osmometer uses the solutions.
















































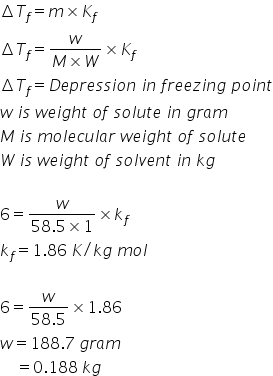











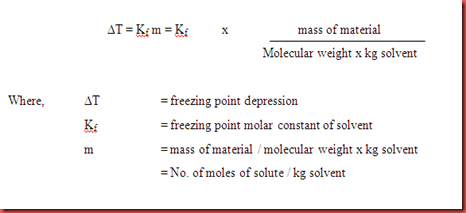

/close-up-of-ice-cubes-against-white-background-603078349-5829fe673df78c6f6a20deff.jpg)